Introduction: In the relentless pursuit of effective treatments and breakthroughs in cancer research, every step forward is significant. Today marks a pivotal moment as SiteCentric, a leading provider of Clinical Trial Management System (CTMS) solutions, announces the commencement of testing at a prestigious European cancer research center site. This partnership heralds a new chapter in streamlining clinical trial operations and accelerating the pace of cancer research.
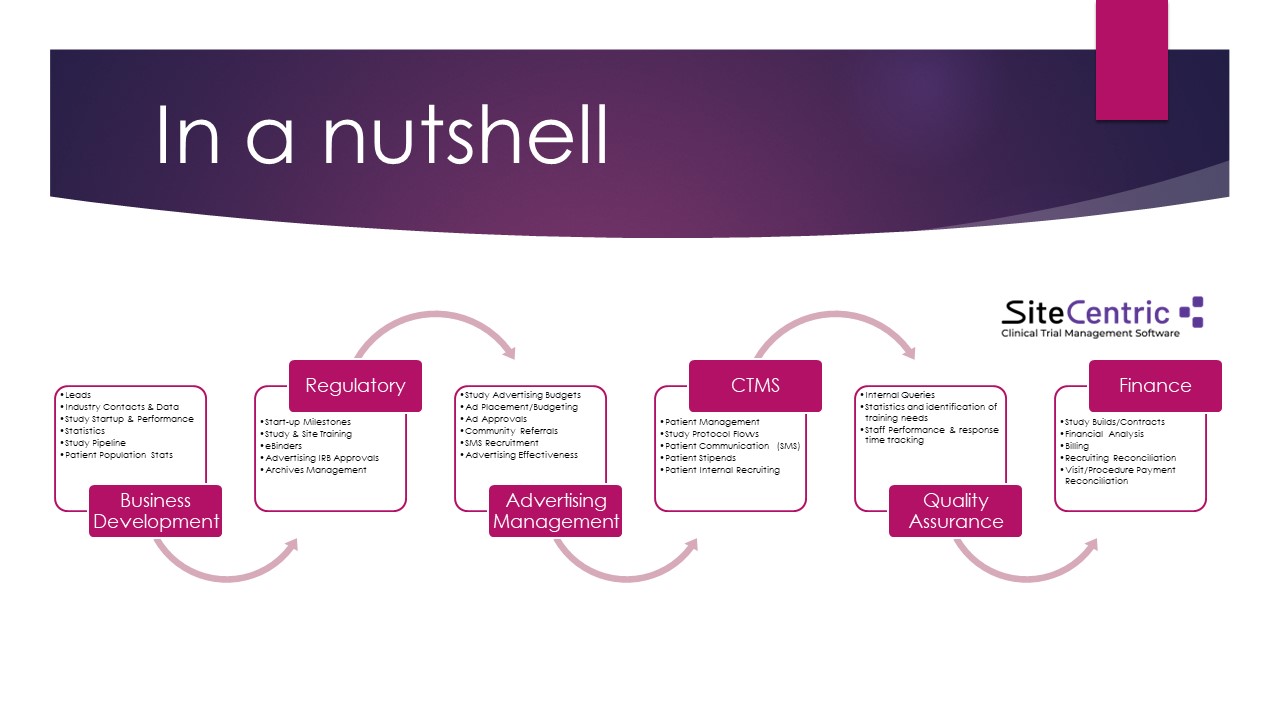
Empowering Research Efforts: Clinical trials are the backbone of medical progress, offering hope and possibilities to patients battling cancer. However, managing these trials efficiently and effectively poses significant challenges. SiteCentric’s CTMS is designed to alleviate these burdens, providing researchers with a comprehensive platform to oversee every aspect of the trial process. From patient recruitment and enrollment to data management and compliance, SiteCentric empowers research teams to focus on what matters most – advancing knowledge and saving lives.
Enhancing Efficiency and Accuracy: At the heart of SiteCentric’s CTMS is a commitment to enhancing efficiency and accuracy in clinical trial management. By digitizing and centralizing trial data, researchers can streamline workflows, reduce administrative overhead, and ensure compliance with regulatory standards. Real-time access to patient data and trial progress enables swift decision-making and proactive problem-solving, ultimately expediting the path from research to treatment.
Collaborative Partnership: The partnership between SiteCentric and the European cancer research center exemplifies a collaborative approach to advancing cancer research. By harnessing the expertise of both parties, this initiative aims to optimize trial operations, foster innovation, and ultimately improve patient outcomes. Through ongoing feedback and iteration, SiteCentric is committed to tailoring its CTMS to the unique needs and workflows of the research center, ensuring seamless integration and maximum impact.
Looking Ahead: As testing commences at the European cancer research center site, excitement abounds for the potential of SiteCentric’s CTMS to drive meaningful change. With each milestone achieved and each obstacle overcome, we move one step closer to a future where cancer is no longer a devastating diagnosis, but a manageable condition. Together, SiteCentric and its partners stand at the forefront of this fight, united in a shared mission to transform cancer care through innovation and collaboration.
Conclusion: In the realm of cancer research, every breakthrough begins with a single step. Today, as SiteCentric’s CTMS testing launches at a prominent European research center site, we take a bold stride forward in the pursuit of a world free from the burden of cancer. With innovation, dedication, and collaboration as our guiding principles, we embark on this journey with unwavering determination and boundless hope for the future. Together, we can – and will – make a difference.
Stay tuned for updates as SiteCentric continues to revolutionize clinical trial management and shape the future of cancer research.