eISF (eBinders) for Research Sites
Electronic Investigator Site File (eBinders) with state-of-the-art remote monitoring capabilities available as a core module at no extra cost.
Integrated
Share data seamlessly between modules
Audit Ready
Utilized successfully on multiple trials audited by both sponsors and FDA
Remote Monitoring
CRA’s and Auditors can access the eISF to remotely monitor the site file
Part 11 Compliant
Documented and Validated Part 11 Compliance
★ ★ ★ ★ ★
I can say, having worked at the site and CRO levels, any site serious about the quality and efficiency of their data, along with the importance of having a cohesive work environment, would take a long hard look at this platform.

Joan Cutillo, CRA II
/
Premier Research
01
Easy Drag-and-Drop Upload
Upload documents easily to eISF and eSource with a simple drag and drop interface.
02
Site Master File
Keep your site’s documents and licenses in one place and link them to eISFs for multiple studies.
Update expiring documents for all active studies simultaneously.
03
Communication Processing
Simply forward an email to the specified eISF address to file both the message and any attachments.
One email can be filed with multiple eISFs with just a few clicks.
04
Remote Monitoring
eISF and eSource modules allow for remote monitoring of regulatory binders and source documentation.
On subsequent logins, a monitor can easily see what has changed since their last review.
05
eSignatures
Allow your staff to electronically review and sign documents in the eISF system.
eSignature workflows allow a document to be signed by users in a specified order.
06
Notifications of Expiring Documents
Don’t miss a beat or run the risk of a document expiring.
SiteCentric® eISF will let you know when a document needs to be updated ahead of time.
Built by sites, for sites
SiteCentric eISF for Sites Highlights
Investigators and auditors alike love the functionality of SiteCentric eISF, while sponsors & CROs benefit from remote monitoring features. Site enjoy the ease of managing their electronic investigator site file that is fully integrated into the regulatory compliance and quality assurance system.
Expiring Documents
SiteCentric eISF keeps track of your expiring documents and alerts you before expiration to update them. Of course, old versions are kept according to GCP.
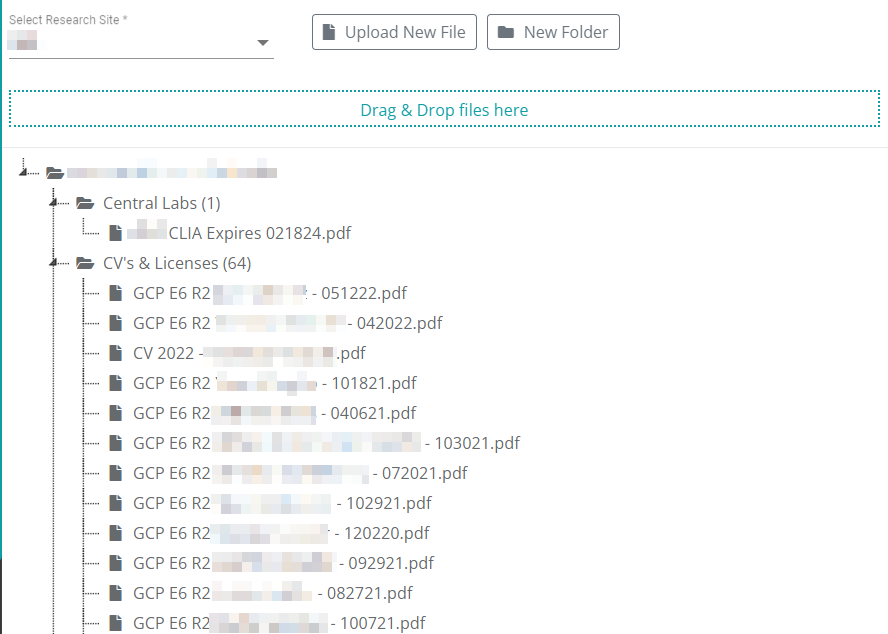
Site Master Documents
Your licenses, site documents, investigator and researcher’s bios, and training records are kept in one place and linked to their relevant studies.
When a document is updated in one place, it can be automatically updated in all linked study binders.
Blockchain Enabled Integrity
While “blockchain” is merely a buzzword for many parts of our industry, this technology is a core feature of the SiteCentric® eSIF module.
Once a document is uploaded, its digital fingerprint is stored in the blockchain. In other words, the authenticity and integrity of the uploaded document can never be questioned as it inherits the blockchain’s immutable properties
This is a must for built-in Part 11 Compliance.
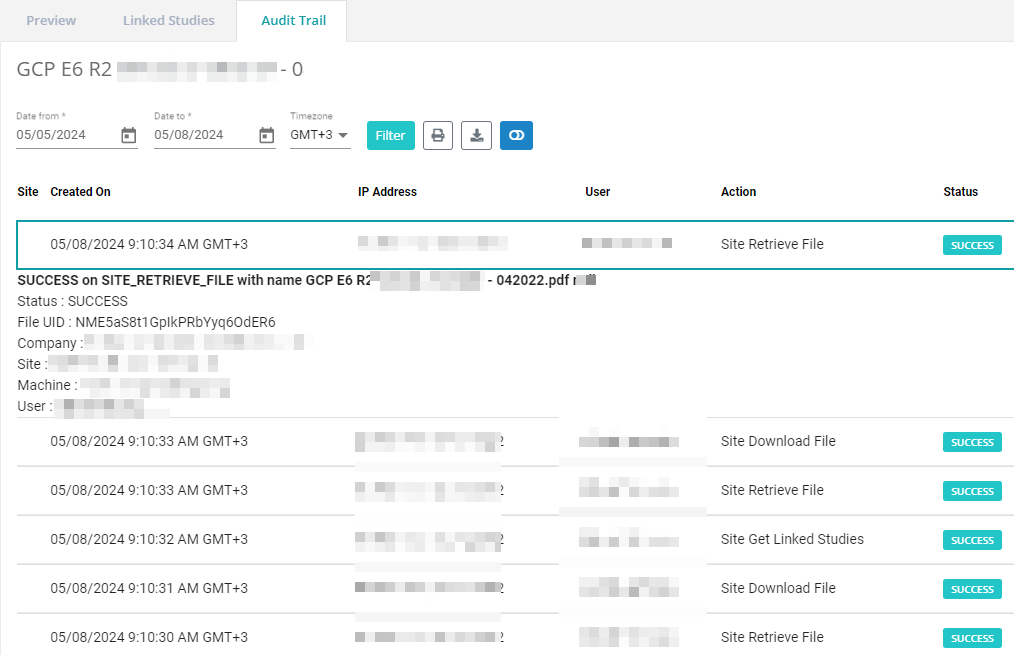
Take the burden out of monitoring and audits!
Let us show you how our integrated eISF can make your site audit ready.
✓ Data Quality/Integrity
✓ Decades if site-specific experience

