Compliance, Regulatory and Quality Assurance for Sites
Powerful Study Start-Up, Clinical Research Compliance, Regulatory
Management and Internal Quality Assurance module as part of the SiteCentric CTMS+ platform
Integrated
with other modules of the SiteCentric® CTMS+ platform
Fast Study Start-Up
Stay on track with regulatory requirements to speed up study startup
Compliance
with regulations, IRB’s and Good Clinical Practice standards
Identify Training Needs
of your clinical teams while reducing risk
★ ★ ★ ★ ★
… The system of checks and balances built into this platform will enable a site to be audit ready at all times! Also, the reduction in protocol deviations will be invaluable as a quality assurance tool…

Joan Cutillo, CRA II
/
Premier Research
01
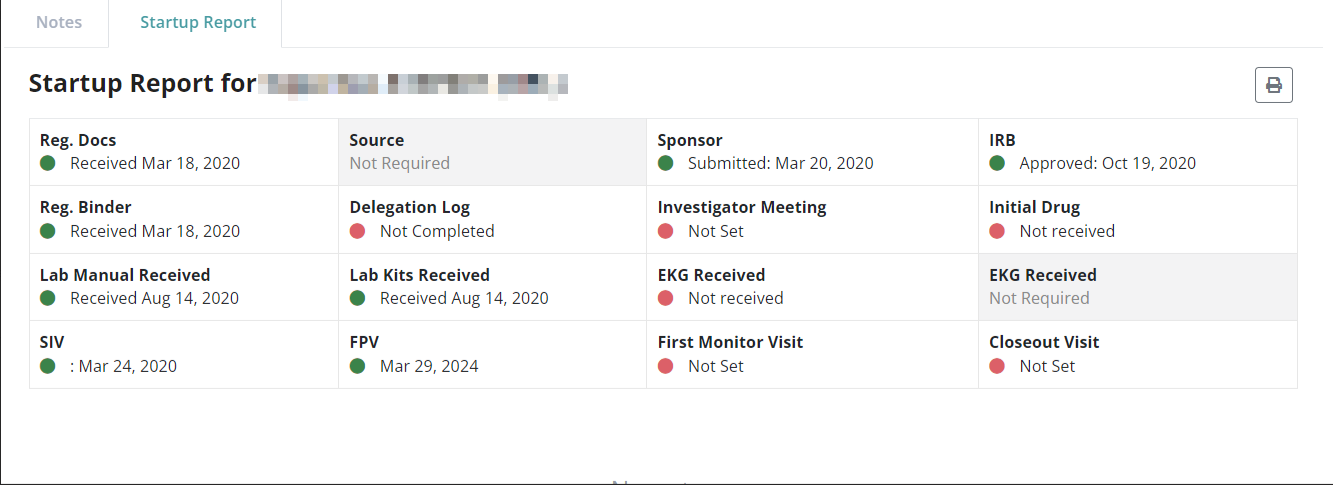
Study Milestones Management
From start-up to closeout, SiteCentric® Regulatory Manager module makes it a breeze managing your study milestones and approvals.
02
Advertising IRB Approvals
Do you still run the risk of running unapproved ads? Integrated with SiteCentric® Patient Recruiting, ad approvals module ensures proper approvals and documentation are secured with safeguards and warnings before an ad is scheduled to run.
Sponsors and CRO’s use QA systems to track your quality and performance. SiteCentric® Quality Assurance offers similar capabilities but for sites. It has the power to identify training needs, and quality gaps to address in order to avoid deviations and increase data quality.
With over 5 audits completed for high enrollment, SiteCentric® eISF is quickly establishing itself as the next leader in the industry. Innovative tools like “Mark Reviewed” and document expirations/notifications make site teams managing their Site File a breeze. Monitors and Auditors love the remote monitoring capabilities and the tools to help them review/download relevant documentation. More here
05
Archives Tracking & Management
While our goal is a complete transition to electronic records in clinical trials, paper remains a significant part of our operation.
SiteCentric® Archives Management utilizes a barcoded system to manage your paper storage (both on-site and off-site), streamlining document retrieval for audits as well as reference, and simultaneously reducing costs.
06
Staff Training Managment
Do you know who has been trained to perform a scale or procedure for a specific study without having to dig up paper?
With SiteCentric’s Training Management tool, integrated into SiteCentric® Regulatory Manager, you can stay on track of training needs and easily identify who can perform the visit or procedure with just a click of a button.
Built by sites, for sites
Regulatory Management & Compliance for Sites
Making regulatory affairs a more enjoyable experience for your users. Taking the complexity out of the process, SiteCentric® Regulatory Manager offers a simple solution to your regulatory affairs and clinical research compliance management.
Keep up with Start-Ups & Training
Effortlessly oversee your study startup milestones and stay updated on project management updates from other departments.
Training stands as a vital component of running a successful clinical trial. It’s not solely about preventing deviations but also crucial for maintaining data quality. SiteCentric simplifies the management of your staff training and study-specific training requirements.
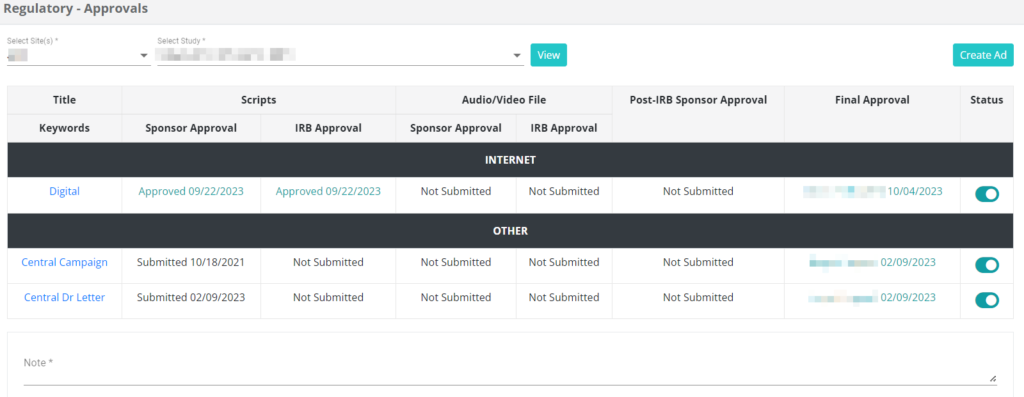
Study-Specific Ad Approvals
By collaborating seamlessly with SiteCentric® Patient Recruitment module, your Regulatory department can actively delegate the Ad approval process for study-specific ads. This ensures adherence to proper approval protocols and safeguards against potential issues stemming from the use of unapproved ads.
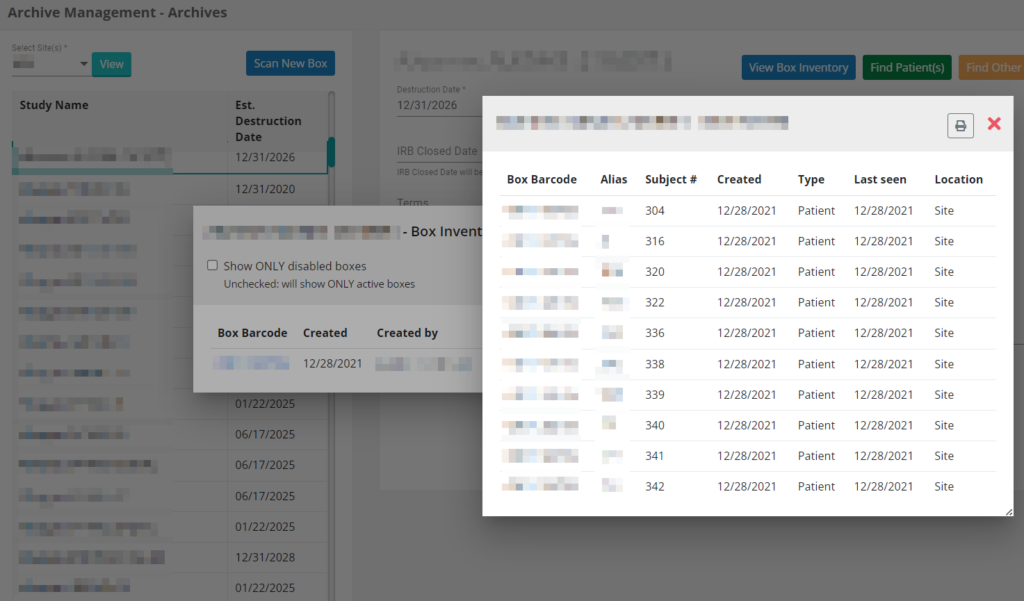
Archives Management
As much as we stride to go fully paperless, the reality is that we all still have paper that needs to be stored in the old fashion way either on-site or off-site.
SiteCentric® Archives Management feature makes it easy to keep track of paper and its location through a barcoded system.
Managing your storage costs can be an important factor. The system keeps track of contractual estimated destruction dates and process for you and flags documents/boxes that could potentially be destroyed to reduce your storage cost.
Regulatory Compliance can be hard, especially with the everchanging landscape of clinical trials.
Tap into our decades of clinical research compliance expertise to help your site operate efficiently and increase quality.
✓ Efficiency at its finest
✓ Decades of site experience
Built by sites, for sites
Quality Assurance for Sites
Simply obtaining a certificate through a test doesn’t encompass the full scope of adhering to Good Clinical Practice (GCP) standards. With SiteCentric’s QA system, you actively monitor your quality, pinpoint training requirements, and mitigate risks effectively.
Monitor Quality
Linked with SiteCentric® CTMS+, you can actively review completed visits for quality. Identifying provider performance and training requirements becomes effortless, enabling informed, risk-based quality assurance decisions.
SiteCentric simplifies the identification of quality deficiencies and facilitates their resolution, ensuring adherence to Good Clinical Practice (GCP) standards.
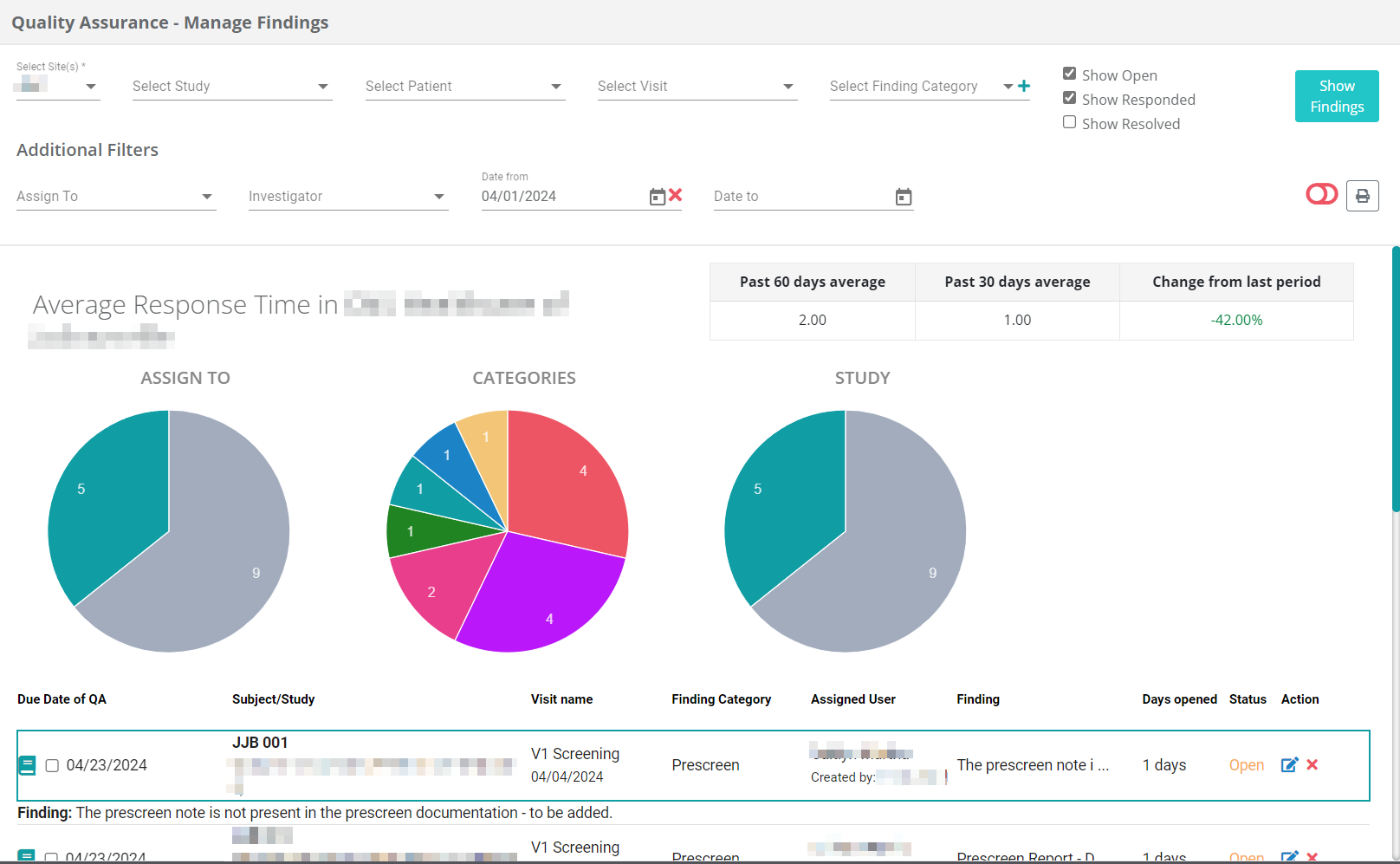
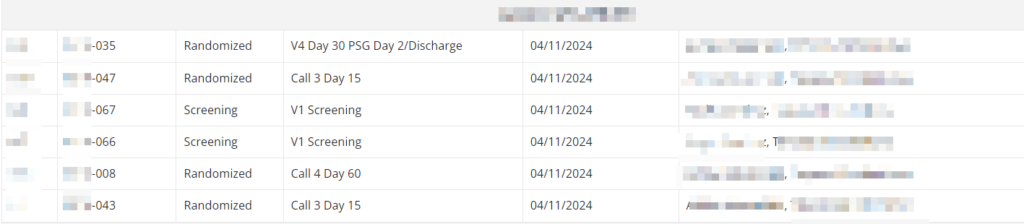
Provider Consistency Tracking
Ensuring consistency in visit conductors is another crucial aspect of maintaining quality. By increasing familiarity, the risk of errors decreases. Our QA modules simplify the identification of discrepancies and enable focused QA efforts in areas of higher risk, ensuring both high data quality and sponsor satisfaction.
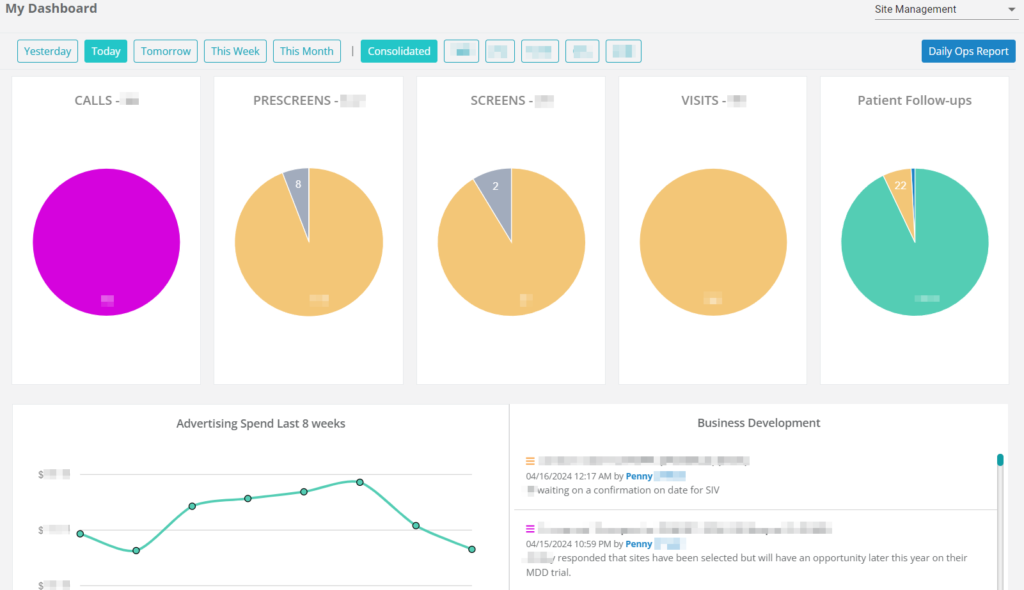
Dashboards & Notifications
Clinicians can directly respond to their findings from their clinical dashboard without having to navigate away from their current tasks or access additional portals for documentation. This fosters increased collaboration with clinical teams and enhances the visibility and significance of quality assurance, leading to improved data quality.
Our CTMS is built and supported by research professionals like you.
Take advantage of our decades of site-level experience in to show you how our platform enables your site to become more efficient, effective and reduce your costs while increasing quality and patient satisfaction,
✓ Efficiency at its finest
✓ Decades of experience

